By Orville Williams
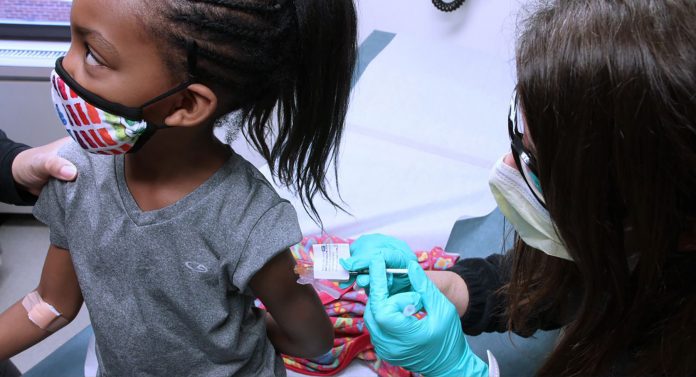
A firm “yes” was the government’s response yesterday, to whether it would make the Pfizer-BioNTech Covid-19 vaccine accessible to children under the age of 12, if US authorities were to approve the use of the vaccine for that age group.
Chief of Staff in the Office of the Prime Minister, Lionel Hurst, apparently did not need more words to communicate his administration’s stance on the matter, as the topic of vaccinations and the need to reduce the spread of the virus again dominated this week’s post-Cabinet media briefing.
The two-dose Pfizer-BioNTech vaccine is currently the only one approved by the US Food and Drug Administration (FDA) for use in individuals as young as 12 years – under Emergency Use Authorisation (EUA) – while it has been given full approval for use in individuals 16 years and older.
Just this week, the vaccine manufacturers issued a joint statement, announcing positive first results from a Phase 2/3 trial of the vaccine in children aged five to 11.
According to the statement, the results showed “a favourable safety profile and robust neutralising antibody responses in children five to 11 years, using a two-dose regimen of 10 micrograms administered 21 days apart, a smaller dose than the 30-microgram dose used for people 12 and older”.
It explained further, that “the antibody responses in the participants given 10-microgram doses were comparable to those recorded in a previous Pfizer-BioNTech study in people 16 to 25 years of age immunised with 30-microgram doses,” adding that “the 10-microgram dose was carefully selected as the preferred dose for safety, tolerability and immunogenicity in children five to 11 years of age”.
According to Pfizer Chairman Albert Bourla, the trial results provide a strong foundation to seek authorisation for use of the vaccine in children aged five to 11, and they plan to submit the data to the FDA and other regulators “with urgency”. He had previously stated the data could be submitted to the FDA by the end of this month.
The FDA, for good measure, said US regulators are “working around the clock” to facilitate the approval of Covid vaccines for children under 12, due to the continued threat.
Dr Peter Marks, the FDA’s top vaccine regulator, even assured last month that his agency would move as quickly as possible to approve the vaccines for that age group, once the companies submit the necessary data.
This is certainly positive news for parents across the region and around the world who have become increasingly aware of the virus’ impact on the younger population, particularly the dangerous Delta variant.
In Antigua and Barbuda, hundreds of parents have already consented to their children aged 12 and over receiving the vaccine, ahead of a planned return to face-to-face learning on October 1.
The Education Ministry reminded those parents and guardians this week that they must give consent for their children to be vaccinated on school compounds, as a student vaccination schedule kicked off on Wednesday.
Even after Pfizer-BioNTech submits its trial data to the US regulators, it is not clear just when the approval might come for the under-12s. In the meantime, the manufacturers will also be releasing clinical trial data on how well the vaccine works in six-month-olds to five-year-olds; that is set to take place as early as the end of October.
